
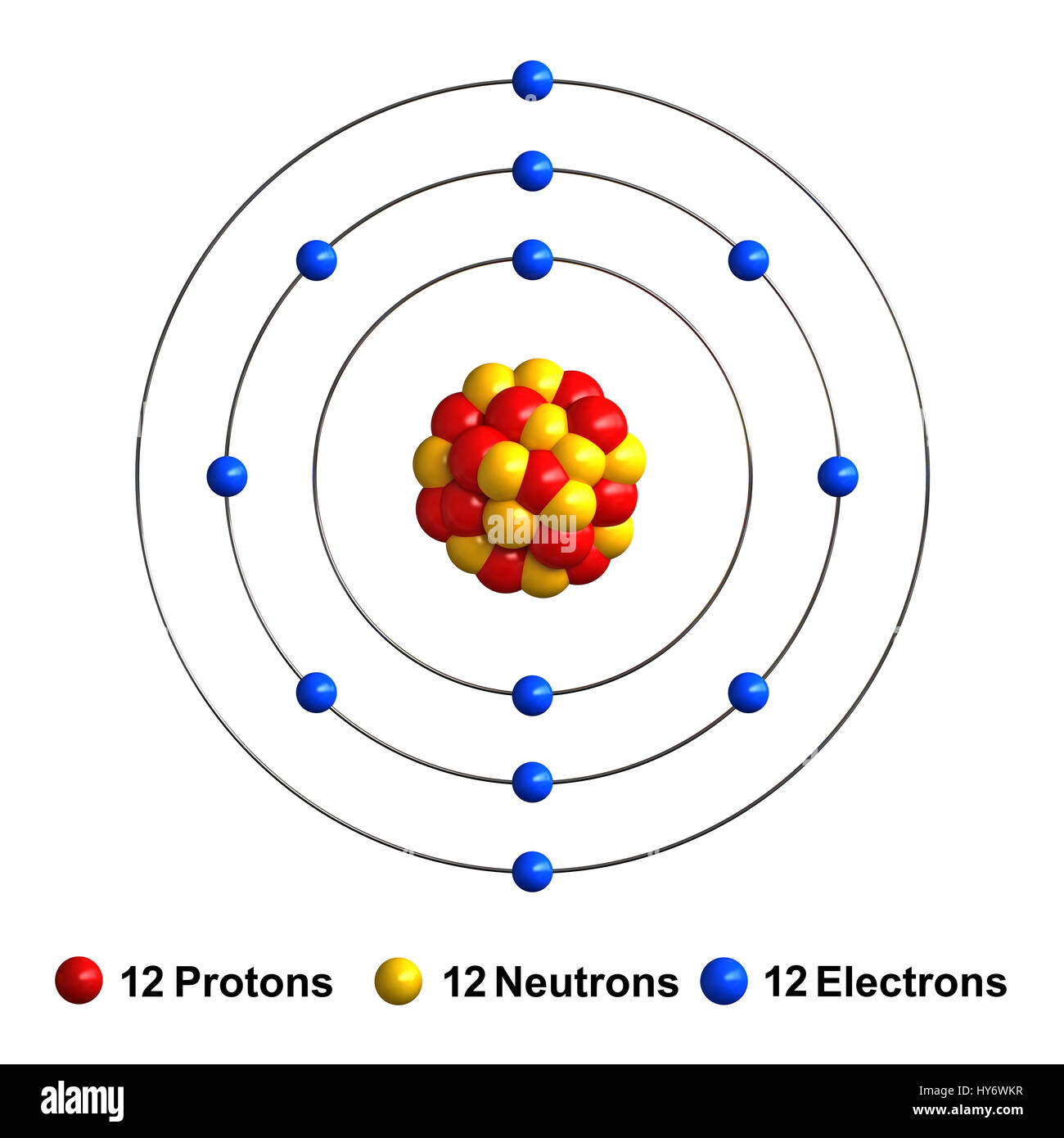
Commercially, magnesium is primarily used in the creation of strong and lightweight aluminum-magnesium alloys, which have numerous advantages in industrial applications. It is can be found in minerals such as brucite, carnallite, dolomite, magnesite, olivine and talc. In its elemental form, magnesium has a shiny grey metallic appearance and is an extremely reactive. Magnesium (12 Mg) naturally occurs in three stable isotopes: 24 Mg, 25 Mg, and 26 Mg.There are 19 radioisotopes that have been discovered, ranging from 18 Mg to 40 Mg (with the exception of 39 Mg).The longest-lived radioisotope is 28 Mg with a half-life of 20.915(9) h. Magnesium is the eighth most abundant element in the earth's crust and the fourth most common element in the earth as a whole. Magnesium was discovered by Joseph Black in 1775 and first isolated by Sir Humphrey Davy in 1808. The magnesium atom has a radius of 160 pm and a Van der Waals radius of 173 pm. The number of electrons in each of Magnesium's shells is and its electron configuration is 3s 2. Magnesium (atomic symbol: Mg, atomic number: 12) is a Block S, Group 2, Period 3 element with an atomic mass of 24.3050. Additional technical, research and safety (MSDS) information is available as is a Reference Calculator for converting relevant units of measurement. Typical and custom packaging is available. American Elements produces to many standard grades when applicable, including Mil Spec (military grade) ACS, Reagent and Technical Grade Food, Agricultural and Pharmaceutical Grade Optical Grade, USP and EP/BP (European Pharmacopoeia/British Pharmacopoeia) and follows applicable ASTM testing standards. Magnesium Metal 26 isotopic material is generally immediately available. 12 electrons (green) bind to the nucleus, successively occupying available electron shells (. For thin film applications it is available as rod, pellets, pieces, granules and sputtering targets and as either an ingot or powder. The nucleus consists of 12 protons (red) and 12 neutrons (blue). Magnesium Metal is also available in ultra high purity and as nanoparticles. Magnesium 26 Metal is one of over 250 stable Metallic isotopes produced by American Elements for biological and biomedical labeling, as target materials and other applications. It is both naturally occurring and a produced by fission. Magnesium 26 Metal (Magnesium-26) is a stable (non-radioactive) isotope of Magnesium. Thin Film Deposition & Evaporation Materials.Additive Manufacturing & 3D Printing Materials.


 0 kommentar(er)
0 kommentar(er)
